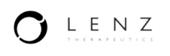- Platform
- Platform Overview
- Life science compliance
- Enterprise-grade security
- Integrations
- Partner Ecosystem
- Workflow management
- Claims & evidence management
- Digital asset management
- Web annotation
- Health authority submission support
- Portals
- Cross channel publishing
- Global commercialization
- Vodori 360
- Reporting & analytics
- Solutions
- Resources
- Company
- Trust
Platform
Platform
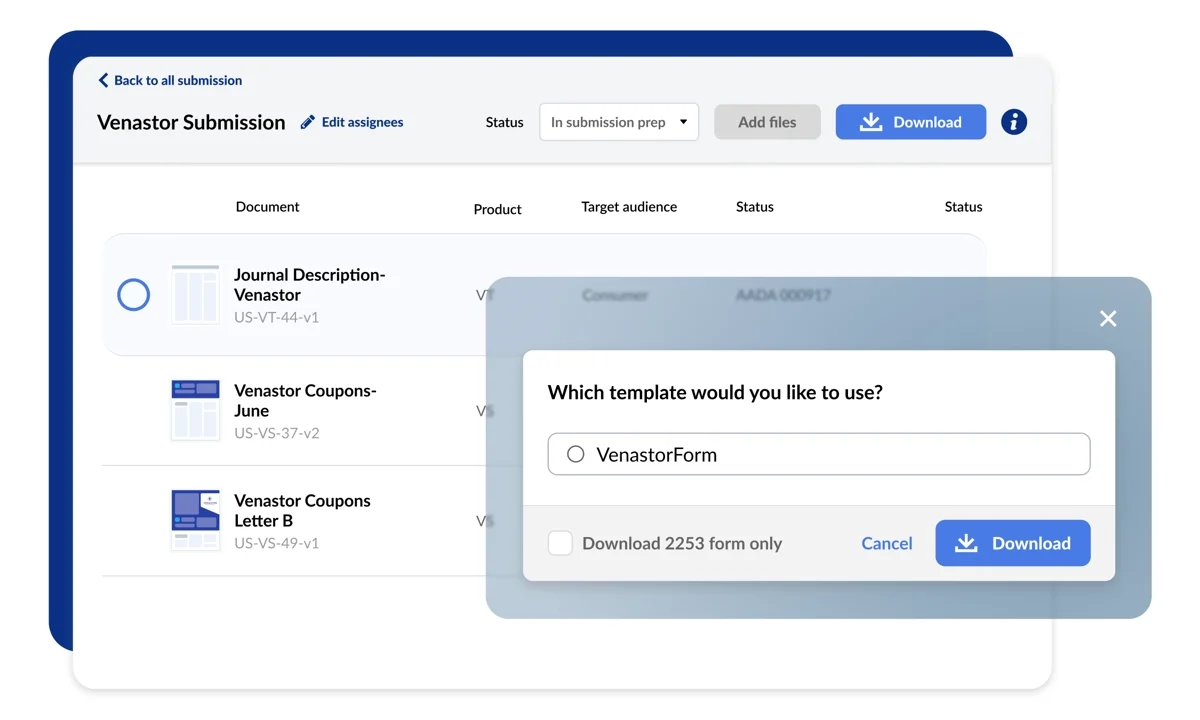
Creation & Approval
-
Workflow management
Manage promotional and medical content, ensure compliance, and collaborate in real-time
-
Claims & evidence management
Substantiate materials with approved claims and references
-
Digital asset management
The source of truth for compliant, on brand materials
-
Web annotation
Review, annotate, and collaborate on digital content in its native format
Distribution
-
Health authority submission support
Prepare materials for health authority submission
-
Portals
Filtered access to approved, curated content
-
Cross channel publishing
Publish approved content from one central, controlled location
-
Global commercialization
Share content across global affiliates
-
Vodori for Salesforce
Deliver MLR approved materials directly to Life Sciences, Sales, and Service Clouds
Solutions
By Business Size






















.png?width=170&height=57&name=Untitled%20design%20(8).png)





-1.png?width=170&height=57&name=sight-sciences-logo@2x%20(1)-1.png)